Background:Patients with R/R CLL/SLL who experience intolerance to or disease progression after Bruton tyrosine kinase inhibitor (BTKi) and venetoclax treatment have no established standard of care and poor outcomes, indicating a critical unmet need. Liso-cel, an autologous, CD19-directed, 4-1BB CAR T cell product, has demonstrated efficacy in large B-cell lymphoma and CLL/SLL. In the primary analysis of the phase 1/2, single-arm, multicenter TRANSCEND CLL 004 (NCT03331198) study, a single administration of liso-cel demonstrated rapid, deep, and durable responses and a manageable safety profile in patients with R/R CLL/SLL, including those with progression on previous BTKi and venetoclax failure (Siddiqi T, et al. Lancet 2023). The primary endpoint was met in the prespecified subset of efficacy-evaluable patients with disease progression on BTKi and venetoclax failure (primary efficacy analysis set [PEAS]) at a target dose of 100 × 10 6 CAR + T cells (null hypothesis: ≤ 5%) with the rate of complete response/remission (CR) and CR with incomplete marrow recovery (CRi) by an independent review committee (IRC) per 2018 International Workshop on CLL (iwCLL) criteria at 18.4% ( P = 0.0006). Here we report results from TRANSCEND CLL 004 with a median follow-up of 23.5 months.
Methods: Patients must have received ≥ 2 prior lines of therapy, including a BTKi. Eligible patients received liso-cel at a target dose of either 50 × 10 6 (dose level [DL] 1) or 100 × 10 6 (DL2) CAR + T cells after lymphodepleting chemotherapy. The primary endpoint was CR/CRi in the PEAS at DL2. Key secondary endpoints were ORR and rate of undetectable MRD (uMRD; 10 −4 by next-generation sequencing) in blood. All null hypotheses were tested at the primary analysis and not retested in this analysis.
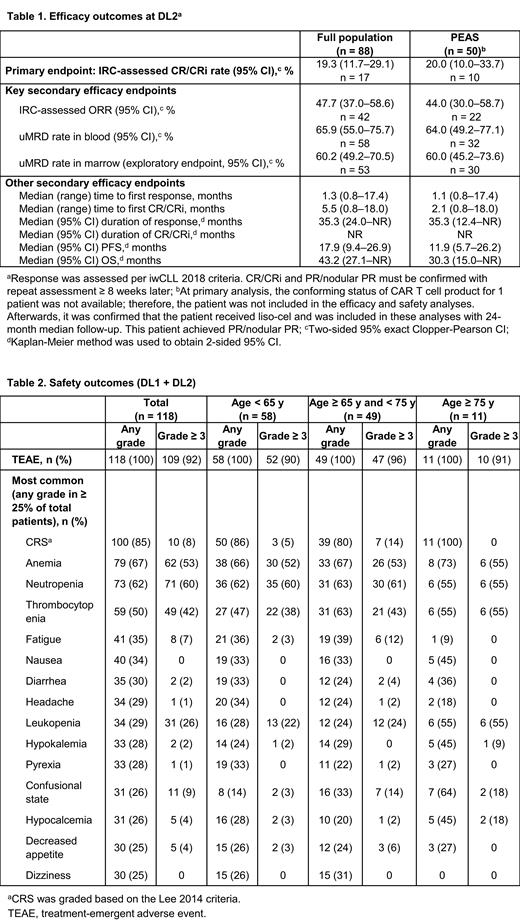
Results:Of 137 leukapheresed patients, 118 received liso-cel (safety set), 97 (DL1 = 9; DL2 = 88) were efficacy evaluable, and 54 (DL1 = 4; DL2 = 50) were in the PEAS. In the safety set, median (range) age was 65 years (49-82), 83% had high-risk cytogenetics (del[17p], 42%; TP53 mutation, 47%; unmuted immunoglobulin heavy-chain variable gene, 47%; ≥ 3 chromosomal aberrations, 61%), median (range) lines of prior therapy was 5 (2-14), and all patients had prior BTKi. As of data cutoff (February 28, 2023), median (range) on-study follow-up was 23.5 months (0.4-59.6) for the safety set. In the PEAS at DL2, CR/CRi rate was 20% (95% CI, 10.0-33.7; Table 1). ORR was 44% (95% CI, 30.0-58.7). One patient who had best overall response (BOR) of partial response/remission (PR) at primary analysis had deepened to CR/CRi at 18 months without any additional therapy. Of 9 patients who had BOR of CR/CRi at the primary analysis, 8 had ongoing CR/CRi and 1 completed the study with the last assessment as CR/CRi. The uMRD rate was 64% (95% CI, 49.2-77.1) in blood and 60% (95% CI, 45.2-73.6) in marrow. Median (95% CI) duration of response was 35.3 months (12.4-not reached [NR]) and median duration of CR/CRi was NR. Median (95% CI) PFS was 11.9 months (5.7-26.2). Median (95% CI) OS was 30.3 months (15.0-NR). The efficacy outcomes were similar in the full population at DL2. Of 16 patients who had BOR of CR/CRi at primary analysis, 10 had ongoing CR/CRi. In the safety set, rates of any-grade and grade ≥ 3 treatment-emergent AEs were similar across age groups (Table 2). The rate of any-grade cytokine release syndrome (CRS) was 85% (grade 3, 8%; no grade 4/5) and neurological events (NE) was 45% (grade 3, 18%; grade 4, 1%; no grade 5); 69% received tocilizumab and/or corticosteroids for CRS/NEs. Median (range) time to onset and resolution was 4 days (1-18) and 6 days (2-37) for CRS and 7 days (1-21) and 7 days (1-83) for NEs, respectively. Prolonged cytopenia (grade ≥ 3 at Day 30 after liso-cel infusion), grade ≥ 3 infections, hypogammaglobulinemia, tumor lysis syndrome, second primary malignancy, and macrophage activation syndrome occurred in 54%, 18%, 15%, 11%, 9%, and 3%, respectively. Forty-five (33%) of 137 leukapheresed patients died after liso-cel infusion (disease progression, n = 27 [20%]; AE, n = 6 [4%]; other reasons, n = 12 [9%]).
Conclusions: With longer follow-up, liso-cel continued to demonstrate durable CR/CRi, high uMRD rates, and a manageable safety profile in patients with heavily pretreated, high-risk R/R CLL/SLL. The safety results from longer follow-up were similar to those reported in the primary analysis with no new safety signals and were consistent across age groups.
Disclosures
Siddiqi:Pharmacyclics, LLC an AbbVie Company: Research Funding; Oncternal: Research Funding; TG therapeutics: Research Funding; Juno therapeutics: Consultancy, Research Funding; Ascentage Pharma: Research Funding; Janssen: Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Maloney:Umoja: Consultancy, Honoraria; Bioline Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board ; Navan Technologies: Current holder of stock options in a privately-held company; MorphoSys: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Fred Hutch: Other: rights to royalties for patents licensed to Juno; Navan Technologies: Consultancy, Honoraria, Other: Member of the Scientific Advisory Board; Novartis: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria, Research Funding; Kite, a Gilead Sciences: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics/BMS, Research Funding; Janssen: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria, Other: Member, Scientific Review Committee, Research Scholars Program in Hematologic Malignancies; Genentech: Consultancy, Honoraria, Other: Chair and Member of the Lymphoma Steering Committee; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board , Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Member of the JCAR017 EAP-001 Safety Review Committee and Member, CLL Strategic Council, Member of the JCAR017-BCM-03 Scientific Steering Committee under BMS, Research Funding; Amgen: Consultancy, Honoraria; A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Other: Member of the Scientific Advisory Board; Chimeric Therapeutics: Other: Member of the Scientific Advisory Board; ImmPACT Bio: Other: Member, Clinical Advisory Board, CD19/CD20 bi-specific CAR-T Cell Therapy Program; Interius: Other: Member, Clinical Advisory Board; Lyell Immunopharma: Other: Member, CAR T Steering Committee. Kenderian:Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding, Speakers Bureau; Mettaforge: Patents & Royalties; Luminary therapeutics: Other: scientific advisory board ; Sendero: Patents & Royalties; Kite/Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno/BMS: Other: Membership on an entity's board of directors or advisory committees, Research Funding; CapstanBio: Consultancy, Other: Scientific advisory board; Torque: Consultancy; Morphosys: Research Funding; LEAHLabs: Consultancy, Current equity holder in private company, Research Funding; MustangBio: Patents & Royalties; Tolero/Sumtomo: Research Funding; Lentigen: Research Funding. Brander:Ascentage: Other: Site PI clinical trial (grant paid to institution), Research Funding; NeWave: Other: Site PI clinical trial (grant paid to institution), Research Funding; DTRM: Other: Site PI clinical trial (grant paid to institution), Research Funding; Pharmacyclics: Consultancy, Other: Site PI clinical trial (grant paid to institution), Research Funding; Catapult: Other: Site PI clinical trial (grant paid to institution), Research Funding; Novartis: Other: Site PI clinical trial (grant paid to institution), Research Funding; TG Therapeutics: Other: Site PI clinical trial (grant paid to institution), Research Funding; Beigene: Other: Site PI clinical trial (grant paid to institution), Research Funding; Juno/Celgene/BMS: Other: Site PI clinical trial (grant paid to institution, Research Funding; AbbVie: Consultancy, Other: Site PI clinical trial (grant paid to institution), Research Funding; ArQule/Merck: Other: Site PI clinical trial (grant paid to institution), Research Funding; MEI Pharma: Other: Site PI clinical trial (grant paid to institution), Research Funding; Genentech: Consultancy, Other: Site PI clinical trial (grant paid to institution), Research Funding; AstraZeneca/Acerta: Other: Site PI clinical trial (grant paid to institution), Research Funding; Pharmacyclics: Other: Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for NCCN panel member CLL/SLL and HCL, informCLL registry steering committee; AbbVie: Other: Core registry steering committee ; CLL Society: Other: Alliance in Clinical Trials: Leukemia committee member & Trial Champion of S1925 . Dorritie:Kite, a Gilead Company: Research Funding; Genentech: Research Funding; Hoffman-LaRoche: Research Funding; Genmab: Research Funding; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Curio and Dava Oncology: Honoraria; Janssen: Research Funding. Soumerai:AstraZeneca, Beigene, Biogen, Bristol Myers Squibb, Roche, Seattle Genetics: Consultancy; Adaptive Biotechnologies, Beigene, BostonGene, Genentech/Roche, GlaxoSmithKline, Moderna, Takeda, TG Therapeutics: Research Funding. Riedell:Intellia Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; MorphoSys: Research Funding; Nkarta: Research Funding; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; CVS Caremark: Consultancy; Calibr: Research Funding; CRISPR Therapeutics: Research Funding; Tessa Therapeutics: Research Funding; Roche: Research Funding; Xencor: Research Funding. Shah:TG therapeutic: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Epizyme: Consultancy; LOXO-Lilly: Consultancy, Other: Travel support; Tundra Therapeutics: Current holder of stock options in a privately-held company; Abbvie: Consultancy; Gilead/Kite: Consultancy; Incyte: Consultancy; Umoja: Consultancy; BMS/Juno: Consultancy; Seattle Genetics: Consultancy; Lilly Oncology: Consultancy, Research Funding; Miltenyi Biotec: Consultancy, Other: Travel support, Research Funding. Nath:Allovir: Consultancy; ADC Therapeutics: Consultancy; Actinium: Consultancy; Incyte: Consultancy. Fakhri:BMS/Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genetech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab/Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO/Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Stephens:AbbVie: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Bristol-Myers Squibb: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Janssen: Consultancy; Lilly: Consultancy; Novartis: Research Funding. Ma:Abbvie: Consultancy, Research Funding; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly and Company/Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genentech: Consultancy. Feldman:Karyopharm: Speakers Bureau; MorphoSys: Speakers Bureau; Secura Bio: Speakers Bureau; Genmab: Consultancy, Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics LLC/Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sankyo: Speakers Bureau; Daiichi: Speakers Bureau; AstraZeneca: Consultancy, Speakers Bureau; ADC therapeutics: Speakers Bureau; Abbvie: Consultancy, Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; Seagen: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; Eisai: Research Funding; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Juno/Bristol Myers Squibb (BMS): Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Honoraria, Speakers Bureau; Kyowa Kirin: Research Funding; Portola Pharmaceuticals: Research Funding; Viracta Therapeutics: Research Funding; Amgen: Research Funding; Pfizer: Research Funding; Trillium Therapeutics: Research Funding; Bayer: Honoraria; Roche: Research Funding; Cell Medica: Research Funding. Schuster:Novartis: Patents & Royalties: Patent for combination therapies of chimeric antigen receptor and programmed cell death protein-1 inhibitors licensed to Novartis; Merck, Genentech/Roche, Novartis: Research Funding; Novartis, Takeda: Honoraria; Caribou Biotech, Genentech/Roche, Genmab, Kite Pharamaceuticals, Incyte, Legend Biotech, Morphosys, Mustang Biotech Nordic Nanovector, Novartis: Consultancy. Perna:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Tuazon:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Ou:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Rane:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Wierda:AstraZeneca/Acerta Pharma: Consultancy, Research Funding; Janssens Biotech Inc: Research Funding; Bristol Myers Squibb (Juno & Celgene): Consultancy, Research Funding; KITE Pharma: Research Funding; Gilead Sciences: Research Funding; NIH P30 CA016672/MDACC Cancer Center Support Grant: Research Funding; GlaxoSmithKline: Research Funding; Genentech: Research Funding; Pharmacyclics LLC: Research Funding; Nurix THerapeutics: Research Funding; Sunesis: Research Funding; AbbVie: Consultancy, Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Cyclacel: Consultancy, Research Funding; Numab THerapeutics: Research Funding; Accutar Biotechnology: Research Funding; Janssens Biotech: Research Funding; Juno Therapeutics: Research Funding; National Comprehensive Cancer Network: Other: Nonrelevant Financial Relationship/Chair, CLL). Supported by the NIH/NCI under award number P30 CA016672 and used MDACC Cancer Center Support Grant (CCSG) shared resources; GSK/Novartis: Research Funding.